

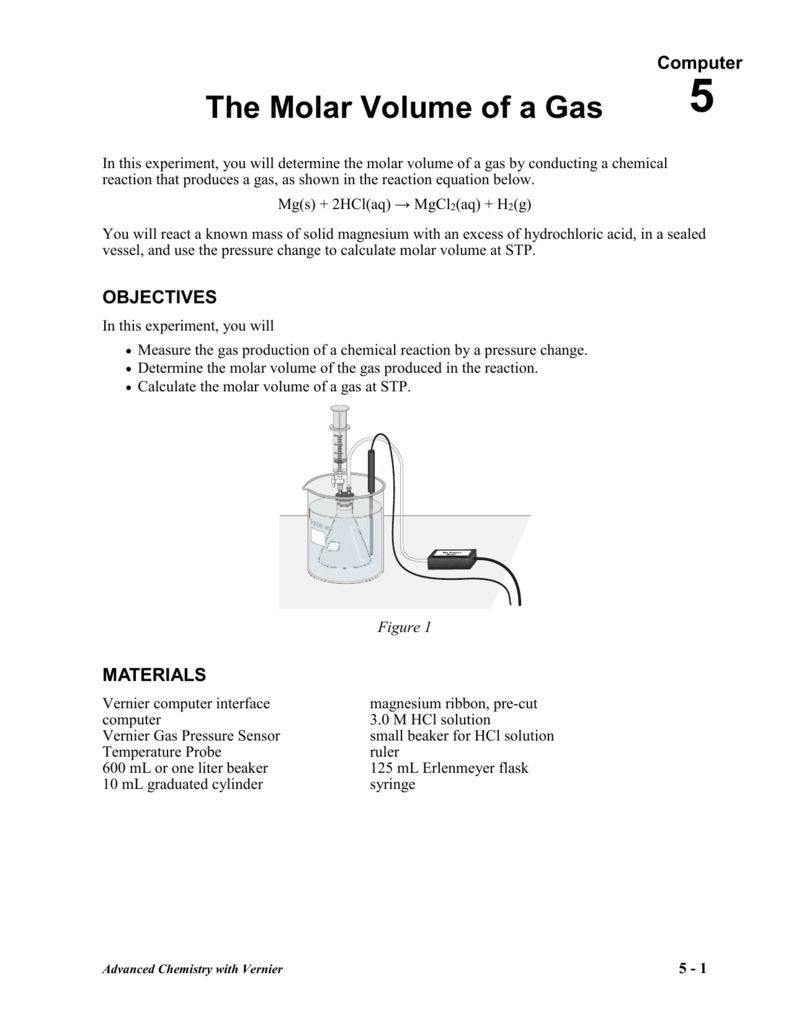
It also recalculates grams per ml to moles. To convert from moles to volume of a substance at STP, standard molar volume (22.4 L/mol) is used as.
MOLAR VOLUME CALCULATOR PROFESSIONAL
Published by McGraw-Hill Professional 1 edition (November 20, 2002). Handbook of Inorganic Chemicals by Pradyot Patnaik (Author).

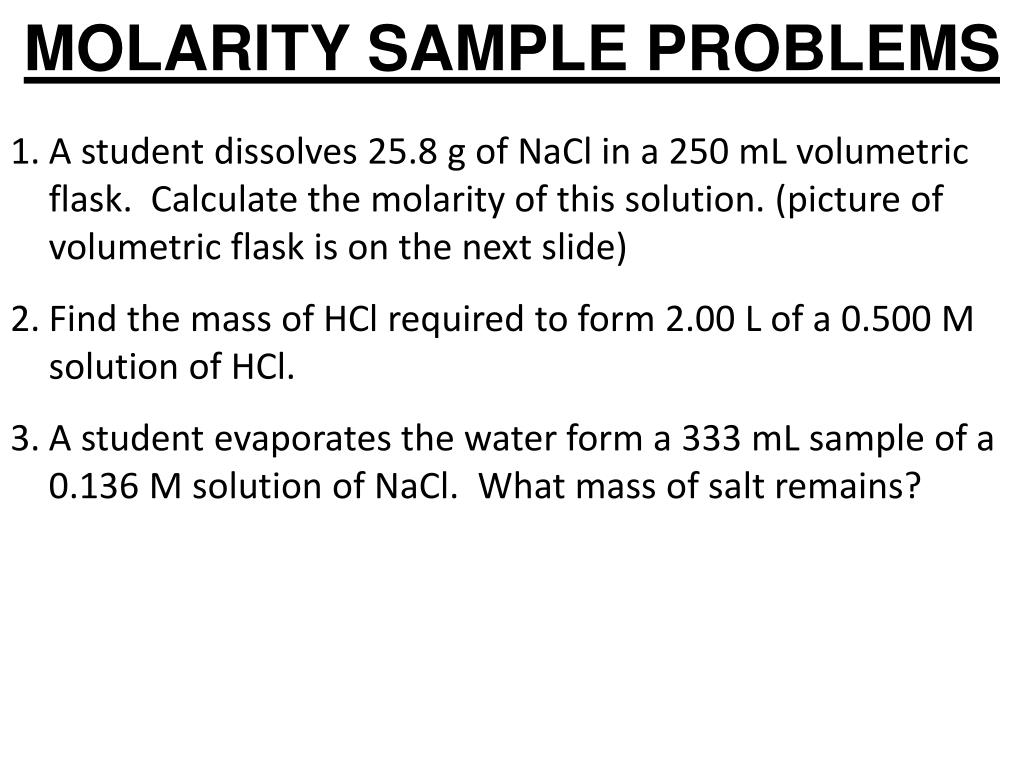
Last accessed: 29 August 2020 (.gov/compound). National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894 USA. National Center for Biotechnology Information U.S. Calcium carbimide weighs 2 290 kg/m³ (142.96003 lb/ft³) Molarity Calculator Calculate the mass, volume or concentration required for a solution.A few materials, substances, compounds or elements with a name containing, like or similar to Calcium Carbonate:.It can find all the values involves in the titration formula. For calculating the molar volumes of gaseous paraffins, the BenedictWebbRubin equation of state can be used by solving it relative to the molar density by the. Also known as: Calcite Calcium Milk Chalk Limestone Marble Marble, solid Milk of Calcium Vaterite.Įlements: Carbon (C), Calcium (Ca), Oxygen (O) Titration calculator is an online chemistry tool used for calculating molarity through titration.Calcium Carbonate is an odorless and tasteless powder or hexagonal crystal. Enter 10 into the Concentration (start) box and select the correct unit (millimolar) Enter 50 into the Concentration (final) box and select the correct unit (micromolar) Enter 20 into the Volume (final) box and select the correct unit (milliliter) Press calculate The answer of 100 microliter (0.Example: Calculate the number of moles of present in 10 dm3 of gas at STP. In Imperial or US customary measurement system, the density is equal to 169.2422 pound per cubic foot, or 1.5671 ounce per cubic inch. In the equation triangle above, the units are litres for all volume measurements. The partial molar of component 1 is the intersection. You can use the third calculator (from page top) to calculate a dilution or preparation of a stock solution. To find the partial molar volumes at some composition x1, draw the tangent line on your curve at x1. It becomes clear that the volume occupied by any number of moles at these conditions can be easily determined: 2 moles 11.6 L/mol 23. density of calcium Carbonate is equal to 2 711 kg/m³ at 25.2☌ (77.36☏ or 298.35K) at standard atmospheric pressure. To get v/v percentage, multiply molarity by molar mass of the substance and divide by 10 times the mass density of the solution. Since molar volume refers to the volume occupied by 1 mole, you'd get V 1 mole 0.082 Latm molK 355K 2.5atm 11.6 L/mol This is how much volume 1 mole occupies at 355 K and 2.5 atm. Calcium Carbonate weighs 2.711 gram per cubic centimeter or 2 711 kilogram per cubic meter, i.e.


 0 kommentar(er)
0 kommentar(er)
